Professor Jui-Hung Hung's Research Team Building the Virtual Epigenome System — EpiVerse
- Published on
- Author
- 張怡婷

Research Team Group Photo
Imagine a DNA strand stretching two meters long, yet intricately folded and packed into a cell nucleus only about 5 to 10 micrometers in diameter—forming a complex "ball of yarn" universe. A research team from the Department of Computer Science at National Yang Ming Chiao Tung University has harnessed artificial intelligence to develop a system called EpiVerse, a novel tool that allows scientists to explore this hidden microcosm within the nucleus in unprecedented ways. Their work has been published in the prestigious journal Nature Communications.
EpiVerse showcases the immense potential of AI in life sciences, particularly in drug discovery and gene regulation modeling. It is reshaping traditional research paradigms that once depended heavily on laborious and costly experiments.
The name "EpiVerse" combines "Epi" from epigenome—which refers to the chemical modifications and chromatin configurations that regulate gene expression beyond the DNA sequence—and "Verse" from metaverse, symbolizing a virtual epigenomic universe created through AI.
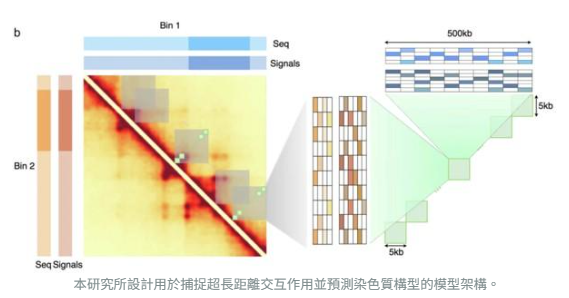
This research introduces a novel model architecture designed to capture ultra-long-range genomic interactions and predict chromatin configurations.
"Traditional studies of chromatin structure relied on complex and expensive experimental procedures. Over the years, our lab has tested various AI models for prediction," said Professor Jui-Hung Hung, the principal investigator of the EpiVerse project.
"Through a carefully designed model architecture, we can now simulate the 3D chromatin structure under different cellular states. The virtual epigenome built by EpiVerse helps researchers better understand gene regulatory mechanisms, opening new analytical pathways for cancer research, drug development, and personalized medicine."
EpiVerse employs multi-stage deep learning and virtual epigenome construction techniques, enabling cross-cell-type prediction. Even when experimental data are limited, EpiVerse can model chromatin structures across different tissues, expanding the scope of gene regulation research.
The team pioneered an architecture that integrates HiConformer, a multi-task transformer model, with a multi-scale image reconstruction network (MIRNet), greatly enhancing both predictive accuracy and visualization quality.
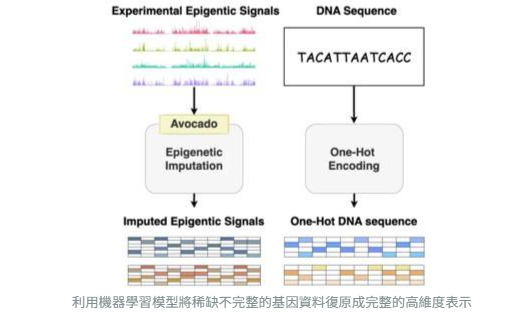
By leveraging machine learning, EpiVerse reconstructs scarce and incomplete genomic data into comprehensive high-dimensional representations.
Another breakthrough feature of EpiVerse is its ability to predict potential chromatin structural changes under varying conditions—such as cellular states, environments, drug treatments, mutations, or cancer metastasis. It enables large-scale in silico perturbation experiments, allowing researchers to simulate and infer gene regulatory networks and potential intervention mechanisms.
This capability provides an unprecedented, systematic approach to understanding the dynamics of chromatin organization, gene regulation, and their relationships to diseases and therapeutics.
"Previously, a single experiment might take months and cost millions, with little room for trial and error. With EpiVerse's simulation power, researchers can now perform multiple perturbation experiments rapidly, obtaining crucial insights for experimental design and vastly improving research efficiency. This represents a tangible realization of AI's computational revolution in the life sciences," said Professor Hung.
Professor Hung also noted that EpiVerse's source code and model are fully open-sourced, providing the global scientific community with a powerful new tool and paradigm for epigenome and chromatin structure analysis.
The project was jointly completed by Professor Hung and two master's students from the Institute of Data Science and Engineering, Yu-Cheng Lo and Ming-Yu Lin, demonstrating NYCU's strong interdisciplinary expertise in AI and bioinformatics talent development.

AI Decoding the "Ball of Yarn" Universe Inside the Cell Nucleus (AI-generated illustration)